
特等奖论文获得者 李慧颖 北京林业大学 副教授
Lactoferrin Suppresses the Progression of Colon Cancer Under Hyperglycemia by Targeting WTAP/m6A/NT5DC3/HKDC1 Axis
Huiying Li
College of Biological Sciences and Technology, Beijing Key Laboratory of Food Processing and Safety in Forestry, Beijing Forestry University, Beijing, P. R. China. E-mail: thufit2012@126.com.
Abstract
Although the relationship between type 2 diabetes (T2D) and the increased risk of colorectal carcinogenesis is widely defined in clinical studies, the therapeutic methods and molecular mechanism of T2D-induced colon cancer and how does hyperglycemia affect the progression is still unknown. Here, a dietary compound lactoferrin is identified as a key factor that alleviates the progression of colon cancer under a high concentration of glucose (like hyperglycemia in T2D) in vivo and in vitro. Mechanistically, lactoferrin-dependent cellular DNA 5mC methylation and RNA m6A profiling remodeling transcriptionally regulate NT5DC3 expression. WTAP plays a key role in regulating NT5DC3 m6A modification and subsequently controls NT5DC3 downstream target HKDC1 expression. Moreover, co-treatment of lactoferrin and NT5DC3 protein restrains the growth of colon tumors by altering the aberrant epigenetic markers. Strikingly, clinical blood samples analysis demonstrates NT5DC3 protein expression is required to direct the distinction of T2D or T2D-induced colon cancer with healthy humans. Together, this study reveals that lactoferrin acts as a major factor to repress the progression of colon cancer under hyperglycemia, thus, significantly expanding the landscape of natural dietary mediated tumor suppression.
Keywords: type 2 diabetes (T2D); colon cancer; NT5DC3; lactoferrin (LF); RNA m6A
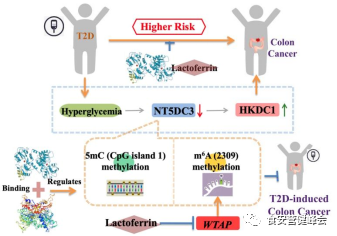
TOC
Introduction
Epidemiological and clinical evidence suggests that type 2 diabetes (T2D) is linked to an increased risk of cancer, especially colon cancer. Several meta-analyses reveal that the patients with T2D have up to 40% higher risks of suffering bladder, breast, colorectal, and kidney cancers than the patients without T2D. Interestingly, research is shedding light on the potential biological links between these two diseases, with some studies suggesting that hyperglycemia might be one of the possible reasons directly linking diabetes and cancer. Hyperglycemic conditions directly reprogram the epigenome, in which DNA hydroxymethylation and protein phosphorylation are mediated as a key switch and regulatory pathway that connects diabetes to cancer. DNA methylation and hydroxymethylation are critical epigenetic processes involved in regulating the expression of tumor suppressor genes, as well as other cancer-related genes that are frequently perturbed in tumor cells. In addition to cancer, alterations in the DNA methylation profile also have been identified in T2D patients. Furthermore, as the most abundant and reversible modification happens at RNA, N6-methyladenosine (m6A) emerges as a new type of epigenetic regulation in recent years. A number of studies have shown that aberrancies in m6A are associated with both cancer and diabetes, and in several diabetic patients, the mRNA level of METTL3 is proved to increase when compared with the healthy volunteers. The integration of DNA/RNA methylation data has further elucidated the pathways of cancer signaling networks. A wholistic insight that highlights and prioritizes DNA 5mC/RNA m6A is required to further unravel the biological complexity of the relationship between T2D and colon cancer.
The coexistence of diabetes and cancer has been found to increase individual mortality, yet some cancer therapeutics can result in transient or permanent damage. The targeting therapies of hormone-based drugs (such as glucocorticoids), tyrosine kinase inhibitors (nilotinib and pazopanib), and mTOR inhibitors (everolimus and temsirolimus), for instance, have been connected to the development of high rates of hyperglycemia. However, antidiabetic therapies, such as metformin, also show implications as targets for the treatment of cancer. Hence, additional studies should be performed to optimize cancer-targeting therapy strategies in patients with diabetes. There is currently increasing interest in the use of natural compounds (diet therapy) to address this issue. One prospective cohort study, based on 136,384 individuals in 21 countries on five continents, reports the association of dairy consumption with a lower risk of mortality, and an increased consumption of dairy products has also been linked to a significant reduction in colon cancer, and the relative risk of T2D is reportedly nearly 10% lower in people with a high milk intake. Thus, whether special bioactive components in dairy products play roles in the progression of T2D-colon cancer, deserves our further research.
Lactoferrin (LF), one of the active biological factors in milk, has recently been considered as a nutraceutical protein. LF is shown to improve the insulin-signaling response, used as an unexpected application in a potential treatment for diabetes. Studies using both in vitro and in vivo models have verified that LF has beneficial effects in cancer treatment, therein suggesting its anti-tumor properties. Recently, the antitumor activities of LF were proved in a human colon tumor model and HT29 tumor-bearing nude mice via the inhibition of tumor angiogenesis and metastasis signal pathways. However, there were few studies that proved lactoferrin with 50% iron saturation suppressed the progression of colon cancer under a high concentration of glucose. Here, we demonstrate the potential advantage of LF (with 50% iron saturation) in the suppression of colon cancer in T2D mice. This study reveals the underlying mechanism by which LF-dependent DNA 5mC and RNA m6A remodeling in epigenetically regulating the NT5DC3/HKDC1 axis, and subsequently inhibiting colon cancer progression under hyperglycemia.
Results
Development of colon cancer in T2D mice model
To set up the in vivo colon tumor model in T2D mice, we conducted a xenograft model screen by using cancer cells derived from different organs, including gastric cancer cell line MGC803, colon cancer cell lines HT29, HCT116, and SW620. The tumor formation time and tumor weight in both T2D and non-diabetic BALB/c nude mice were determined. Though the diabetic mice were found to be at a comparatively higher risk of developing tumors, there were significant differences in the tumor formation (Figure 1A-B, Supplementary Figure S1A, in original article), and the HT29 cells were used for the following in vitro and in vivo studies.
As the closed links among diabetes, hyperglycemic, and colon cancer, the cellular glucose concentration could be critical in the progression of colon cancer. By assessing cell viability and cell apoptosis assays, 2 g·L-1 (11.1 mM) and 5 g·L-1 (27.8 mM) were selected as the normal glucose and high glucose concentrations for the following experiments, respectively (Supplementary Figure S1B and S1C, in original article).
As shown in Supplementary Figure S1D and S1E (in original article), LF treatment showed obviously suppressive ability in migration and invasion activities of HT29 cells (comparing with the control, P < 0.05), further validating the potential anti-malignancy effect of LF on colon cancer. Taken together, we screened HT29 colon cancer cells as the working model for colon cancer development under a high concentration of glucose (hyperglycemia) and verified the potent function of LF.
High throughput sequencing identified NT5DC3 as the key factor in the progression of colon cancer under a high concentration of glucose
To evaluate the inner mechanism of the progression of colon cancer under a high concentration of glucose, we performed high throughput RNA sequencing and DNA methylation detection to screen potential candidates that involved in colon carcinogenesis . We analyzed that 5'-nucleotidase domain-containing protein 3 (NT5DC3) might be a potential biomarker in colon tumor progression under hyperglycemia (Figure 1C and Supplementary Figure S2, in original article).
NT5DC3 and HKDC1 are regulated by LF
Hexokinase domain component 1 (HKDC1) is reported to play a critical role in the maintenance of glucose homeostasis and there is increasing evidence to suggest that its overexpression may contribute to several types of cancers. To this end, we examined the mRNA and protein levels of NT5DC3 and HKDC1 under normal or high glucose conditions in different types of cells, and found that the important mechanism underlying LF’s anti-tumor activity via the regulation of NT5DC3 and HKDC1 (Figure 1J, 1K).
LF inhibits colon tumor development in T2D mice
As the LF-dependent NT5DC3/HKDC1 expression alteration in colon cancer, we sought to determine the relevance of LF and downstream targets NT5DC3/HKDC1 in HT29 tumor model in vivo. To this end, we assessed the HT29 implantation tumor model in C57BL/6 mouse and BALB/c nude mice. These results provided evidences that the high risk of diabetic mice in developing colon cancer could be suppressed by LF and NT5DC3 (Figure 2).
LF suppresses 5mC and m6A of NT5DC3
To elucidate the epigenetic regulation of NT5DC3 in the progression of colon cancer under hyperglycemia condition, DNA 5mC of NT5DC3 on genome level and RNA m6A modification of NT5DC3 on transcript level were detected. The ratio of DNA 5mC/C or RNA m6A/A was upregulated under a high glucose treatment in comparison to normal glucose conditions (lane 1 vs. 3) and downregulated after the transfer from high to normal glucose conditions (lane 3 vs. 5), and there were significant statistical differences. Further, we found the knockdown of NT5DC3 had no obvious effect on the ratio of DNA 5mC/C or RNA m6A/A, further indicating NT5DC3 was the downstream of LF-dependent regulation. These data demonstrate the epigenetic regulation of cellular glucose concentration and LF is mediated by SAM/SAH dependent DNA 5mC or RNA m6A proportion, suggesting a high concentration of glucose-induced DNA or RNA methylation could be alleviated by LF treatment (Figure 3).
The mechanism of the epigenetic regulation of NT5DC3 by LF
DNMT level was upregulated under a high glucose treatment which could be inhibited by LF . In conformity with this result, the NT5DC3 CpG island 1 level was changed at the same pattern. All the eight m6A-related genes (METTL3, METTL14, WTAP, FTO, ALKBH5, YTHDF1, YTHDF2 and YTHDF3) were observed to change significantly under a high glucose in comparison to the normal group, the levels of m6A eraser genes (FTO and ALKBH5) were downregulated, the levels of m6A writer genes (METTL3, METTL14 and WTAP) and m6A reader genes (YTHDF1, YTHDF2 and YTHDF3) were all upregulated under the high glucose conditions, indicating that m6A was activated under hyperglycemia. However, LF could upregulate the levels of m6A eraser genes and downregulate the levels of m6A writer genes and reader genes under the high glucose conditions, but not under normal glucose conditions, further verifying that LF selectively decreased the degree of m6A under hyperglycemia. Furthermore, the RNA m6A of NT5DC3 level was obviously upregulated under a high glucose in comparison to the normal group, and LF significantly attenuated the NT5DC3 m6A level at site 2309. These results demonstrate LF could affect the 5mC and m6A machine genes’ expression to control the methylation status of NT5DC3 (Figure 4, in original article).
The epigenetic regulation of NT5DC3 and potential consequences in vivo
We further investigated the epigenetic regulation of NT5DC3 in vivo, and found that regulation of NT5DC3 by 5mC and m6A is conserved from mice to humans, underlying the importance of this instinct regulation mechanism in vivo (Figure 5, in original article).
Discussion
In this study, we found NT5DC3 as a key target of diabetes and colon cancer under hyperglycemia. Our identification and development of colon cancer cells induced mouse tumor models provide a platform to investigate the effect of natural dietary component LF. On the other hand, based on the co-analysis of transcriptomics and DNA methylation detection, we defined NT5DC3 as the tumor suppressor in inhibiting colon cancer and preliminarily identified the regulation of LF on NT5DC3.
Several studies have demonstrated that LF can be applied as a candidate treatment for diabetes or to efficiently inhibit the growth of tumors and reduce susceptibility to cancer. Notably, the methylation of the promotor and the first intronic region of LF, associated with an increased incidence of cancer, has been observed in cancers of the breast, lung, prostate, etc. Alterations of the methylation profile have been described in T2D, however, information regarding the relationship between LF, methylation, and T2D remains limited. In the present study, LF was found to decrease 5mC and m6A methylation of NT5DC3 in colon tumor cells and xenografted colon tumors implanted in T2D mice, thereby suggesting its potential to suppress the progression of colon cancer under a high concentration of glucose (hyperglycemia).
By using epigenetic and biochemistry techniques, we successfully discovered and verified the modification sites of NT5DC3 regulated by LF. LF was proved to destroy the bridge between T2D and colon cancer through the suppression of epigenetic modification levels of 5mC (CpG island 1) and m6A (2309) of NT5DC3. We also found that WTAP played a key role in m6A (2309) of NT5DC3, which subsequently affect the expression levels of NT5DC3/HKDC1. Our study uncovered that WTAP participated in m6A (2309) of NT5DC3 to suppress NT5DC3 expression thus, LF-dependent WTAP expression inhibition exhibited a novel pathway to suppress the progression of colon tumor under a high concentration of glucose. However, how LF inhibits WTAP expression needs further elucidation. These discoveries about the mechanism of NT5DC3 in suppressing colon cancer progression under hyperglycemia, combined with the positive regulation of LF on NT5DC3, will expand current knowledge of the functions of bioactive proteins within different research layers.
In brief, we revealed that lactoferrin acts as a suppressor in the progression of colon cancer under a high concentration of glucose, by which epigenetically regulating glucose-dependent DNMT/5mC or WTAP/m6A/NT5DC3/HKDC1 axis. Lactoferrin could regulate WTAP and inhibit m6A of NT5DC3 at 2309 site, and subsequently affect the expression levels of NT5DC3/HKDC1 proteins in vitro and in vivo.
Materials and Methods
Please refer to “Lactoferrin Suppresses the Progression of Colon Cancer Under Hyperglycemia by Targeting WTAP/m6A/NT5DC3/HKDC1 Axis” in “Journal of Translational Medicine”.
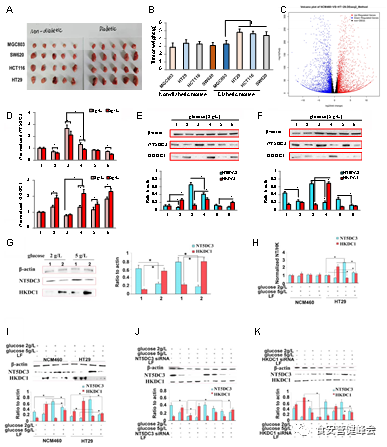
Figure 1 The effect of LF in regulating NT5DC3 and HKDC1.
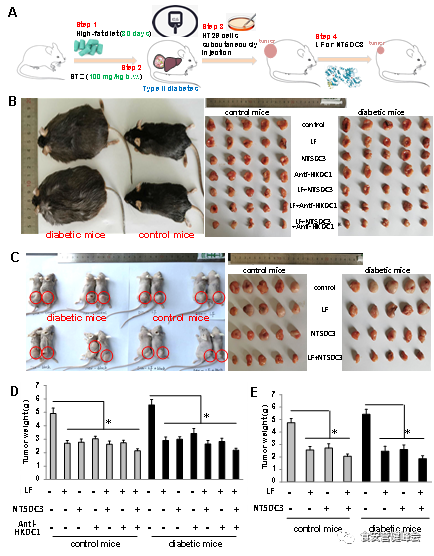
Figure 2. HT29 xenograft tumors in two mouse models.
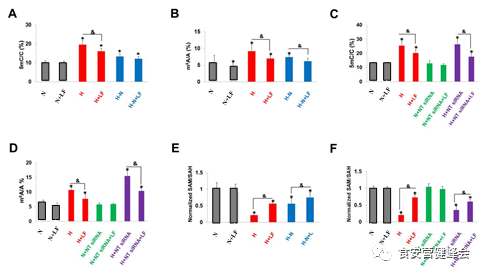
Figure 3 The total 5mC and m6A levels, as well as SAM/SAH ratio detected by MS.
